RESEARCH ARTICLE
Hydrazonoyl Halides: Their Versatile Biological Activities
Ahmad S.Shawalia, *, Nevien A.Samyb
Article Information
Identifiers and Pagination:
Year: 2009Volume: 2
First Page: 8
Last Page: 16
Publisher Id: TOBCJ-2-8
DOI: 10.2174/1874847300902010008
Article History:
Received Date: 21/10/2008Revision Received Date: 10/11/2008
Acceptance Date: 2/12/2008
Electronic publication date: 29/1/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
The various biological activities namely anthelmintic, antiarthropodal, antiviral, antimicrobial, herbicidal, antisarcoptic, acaricidal, insecticidal and miticidal activities exhibited by the hydrazonoyl halides are surveyed. Also, the uses of such halides as pesticides, weed controlling and antihypertensive agents as well as lipoxygenase and cyclo-oxygenase inhibitors are presented. Furthermore, their contact dermatitis and phytotoxicity effects are pointed out in addition to their metabolic fate.
1. INTRODUCTION
Hydrazonoyl halides 1 are those compounds which have the characteristic function -C(X):NNH-, where X is a halogen group (e.g. Br or Cl). They are structurally related to hydrazonoic acids 2 in the same way as the imidoyl halides 3 are related to imidoic acids 4.
1, RC(X)=NNHR’ 2, RC(OH)=NNHR'
3, RC(X)=NR' 4, RC(OH)=NR'.
The first hydrazonoyl halide was described by Fisher shortly before the begining of the 20th century [1]. Since that time an increasing flow of work has appeared on the chemistry of such a class of compounds so that the output of published work recorded a peak of 261 papers and 53 patents in the eighties. The interest in the chemistry of such halides is a consequence of the fact that they undergo a wide variety of reactions which provide routes to a myriad of both heterocyclic and acyclic compounds. In addition, diverse biological activities such as anthelmintic, antiviral, antiarthropodal, antimicrobial, fungicidal, herbicidal, antisarcoptic, insecticidal, pesticidal, acaricidal, miticidal, etc., have been found to be associated with hydrazonoyl halides. In recent years, interest in the chemistry of this class of compounds has been renewed because of the development of novel synthetic routes and their use as versatile synthons for other compounds that found many applications in both industrial and pharmaceutical fields. At present there are ten review articles by Shawali et al. [2-1] covering the various aspects of the chemistry of such halides and their utility in synthesis of heterocycles, Another review by Butler and Scott outlined intermolecular and intramolecular substitution reactions of such halides [12]. In addition, an earlier summary dealing with the chemistry of these compounds had been incorporated by Ulrich in his review of imidoyl chlorides that appeared in 1968 [13]. No comprehensive review on the various biological activities of hydrazonoyl halides has appeared hitherto.
The goal of the present review is to bring to the reader’s attention the scope of the biological activities of hydrazonoyl halides. The literature is covered from 1968 up to mid of 2008. During this period more than 1750 articles and 150 patents making reference to the chemistry and applications of the title compounds have appeared. We hope that this review will stimulate the interest of both chemists and biologists in exploring further the chemistry of such compounds and their biological applications.
2. NOMENCLATURE
The nomenclature applied to hydrazonoyl halides has over the years been somewhat confusing. For example, the following compound was cited in literature under the names indicated below:
C6H5C(Cl)=NNHC6H5
α-Chlorobenzylidene phenylhydrazine
Benzoyl chloride N-phenylhydrazone
N-Phenyl benzhydrazidoyl chloride
α-Chlorobenzaldehyde phenylhydrazone
N-Phenyl benzenecarbohydrazonoyl chloride.
In this review, it is intended to adhere to the nomenclature rules adopted by Chemical Abstracts. According to the latter, the name of a given hydrazonoyl halide, in the absence of higher function or more preferred compound class, is derived from the parent acid by functional group replacement nomenclature. The suffixes appended to the names of molecular skeletons of carboxylic acid, hydrazonoic acid and the corresponding hydrazonoyl halide are as follows:
| GeneralFormula | RC(OH)=O | RC(OH)=NNH2 | RC(X)=NNH2 |
| Suffix | Oic Acid | Hydrazonoic acid | Hydrazonoyl halide |
| Carboxylic acid | Carbohydrazonoic acid | Carbohydrazonoyl halide |
To illustrate the application of these rules of nomenclature, the following examples are given:
CH3C(Br)=NNHC6H4NO2-4
N-(4-Nitrophenyl) ethanehydrazonoyl bromide
4-BrC6H4C(Cl)=NNHC6H5
N-Phenyl 4-bromobenzenecarbohydrazonoyl chloride
CH3COC(Cl)=NNHCOC6H5
N-Benzoyl 2-oxopropanehydrazonoyl chloride
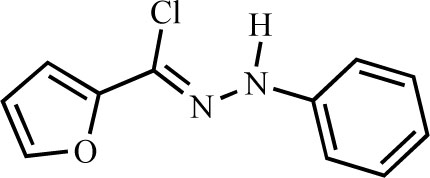
N-Phenyl furan-2-carbohydrazonoyl chloride
In the presence of other groups that have priority to hydrazonoic acid group, the hydrazonoyl halide is named as a hydrazono derivative of the parent compound and this is illustrated by the following two examples.
HOOC-C(Cl)=NNHC6H5
N-Phenylhydrazono-chloroacetic acid
C2H5OCO-C(Br)=NNHCONH2
Ethyl N-[(aminocarbonyl)hydrazono]bromoacetate
Also, when the hydrazonoyl halide residue (-C(X):NNH-) is a part of a more preferred class, it is named as halo derivative of the parent compound of such a class. For example, the halide (C6H5N=N-C(Cl)=NNHC6H5), as it belongs to the class of formazans (R-N=N-CH=NNHR') which is ranked highest of all nonfunctional nitrogen compounds, below imines but above nitrogen heterocycles, it is thus named as 3-chloro-1,5-diphenylformazan.
The various types of hydrazonoyl halides whose biological effects were explored are listed in Table 1.
| No. | structure of Hydrazonoyl halide | No. | General structure of Hydrazonoyl halide |
| I | Ar-C(X)=NNHAr` | VIII | ROCO-C(X)=NNHAr` |
| II | Ar-C(X)=NNHSO2Ar` | IX | ArNHCO-C(X)=NNHAr |
| III | R-C(X)=NNHAr | X | (RO)2P(O)-C(X)=NNHAr |
| IV | Het-C(X)=NNHAr | X1 | R-(C(X)=NNHAr)2 |
| V | HCO-C(X)=NNHAr | XII | (ArNHN=C(X))2 |
| VI | RCO-C(X)=NNHAr | XIII | NC-C(X)=NNHAr |
| VII | ArCO-C(X)=NNHAr |
3. BIOLOGICAL ACTIVITIES
The patent literature contains a large number of hydrazonoyl halides that were reported to have various biological effects. Because of the diversity of structures covered, the discussion is presented according to the biological activity rather than chemical structure of the hydrazonoyl halide.
3.1 Contact Dermatitis
N-Phenyl benzenecarbohydrazonoyl chloride IA was reported to cause severe dermatitis in human [14]. Out of 6 people, five-chemistry students were affected following a single contact. The clinical picture of the dermatitis was characterized by a biphasic course. Circumscribed slight lesions, developing usually within one day in directly affected areas were followed after 4 to 12 days by generalized widespread erythema and edema with papules and vesicles [14].
IA, Ar-C(Cl)=NNHAr', Ar = Ar' =C6H5
3.2. Anthelmintic Activity
N-phenyl 4-methylbenzenecarbohydrazonoyl chloride IB was the first candidate of hydrazonoyl halides that proved effective against gastrointestinal nematodes and cestodes of ovines following a single oral dose of 30-50 mg/kg [15-19]. The sheep developed mild diarrhea and anorexia 10 days after treatment which lasted for 2-4 days, after which the sheep acquired their normal health. No ill signs were observed one week after treatment. N-(4-Chlorophenyl) 4-methoxybenzene-carbohydrazonoyl chloride IC has also been found to be an effective potent anthelmintic compound [20].
IB 4-MeC6H4C(Cl)=NNHC6H5
IC 4-MeOC6H4C(Cl)=NNHC6H4Cl-4
In attempt to elucidate the relationship between structure and anthelmintic activity (SAR), Rector and coworkers [21] prepared a series of thirty substituted N-phenyl benzenecarbohydrazonoyl chlorides I and evaluated their activity against three mouse helminths, Syphacia obvelata, Nematospiroides dubius and Hymenolepis nana. The dosage used was the highest dose (up to 7.5 mg / mouse / day) which did not elicit toxic effects on the mouse. The structural features required to give the superior anthelmintic activity in the series studied are meta- and/or para- halogen, alkoxy, alkyl, or alkylthio substituent(s) in the acid ring moiety. This is because any compound with an o-substituent in the acid ring residue was found to lack activity. Except for the N-(p-chlorophenyl), any substituent in the N-ring residue gave compounds with little or no activity. Multiple substitution in either ring decreases the activity. Also, the reported data revealed that there did not appear to be the detrimental ortho effect operating in the N-ring substitution as was apparent in the acid ring case.
The anthelmintic activity of other series of N-aryl and N-alkyl benzenecarbohydrazonoyl bromides IB (X= Br) and their chloride IB' (X = Cl) analogs were tested on mice infected with the nematode Nippostrongylus braziliensis [22, 23]. The most active compound was N-(2-bromo-4-nitrophenyl) benzenecarbohdrazonoyl bromide IBc and the least active was N-(4-methylphenylsulfonyl) benzenecarbohydrazonoyl chloride IBd.
IBc C6H5C(Br)=NNHC6H3Br(NO2)-2,4
IBd C6H5C(Br)=NNHSO2C6H3Me-4
Other derivatives such as N-phenyl 4-methylthiobenzene-carbohydrazonoyl chloride IB and its N-(4-methylthio) benzenecarbohydrazonoyl chloride IC as well as N-(2,4-dibro-mophenyl) 4-methylthiobenzenecarbohydrazonoyl chloride ID were reported to be useful anthelmintics [24].
IB-D ArC(Cl)=NNHAr'
Ar / Ar': B, 4-MeSC6H4 / Ph; C, Ph / 4-MeSC6H4;
D, 4-MeSC6H4 / 2,4-Br2C6H3
Also, N-phenyl derivatives of 4-methyl-, 4-nitro- and pentafluoro- benzenecarbohydrazonoyl chlorides as well as N-(2,4-dibromophenyl) benzenecarbohydrazonoyl chloride and N-(2,4,6-trichlorophenyl) 4-chlorobenzenecarbohydra-zonoyl chloride were reported to be useful anthelmintics [25].
IB-D Ar-C(X)=NNHAr'
Ar / Ar': IB, C6H5 / 2,4-Br2C6H3;
ICa, 4-MeC6H4 / Ph; ICb, 4-O2NC6H4 / C6H5;
ICc, C6F5 / C6H5;
ID, 4-ClC6H4 / 2,4,6-Cl3C6H2
Moon et al.[26] synthesized a few N-aryl 2-oxopro-panehydrazonoyl chlorides VI in search of new anthelmintics. The results showed that N-(2,4-dichlorophenyl) derivative VIa (250 mg/Kg orally) was effective in sheep and N-(2-chloro-4-nitrophenyl) derivative VIb (30 mg/Kg orally) was effective in dogs.
VI CH3COC(Cl)=NNHAr
Ar: a, 2,4-Cl2C6H3; b, 2-Cl,4-O2NC6H3
Some N-aryl C-heteroarylmethanehydrazonoyl chlorides IV were also reported to be useful as anthelmintics [27, 28].
IV Het-C(Cl)=NNHAr
Het: A, 2-furyl; B, 5-Br-2-(5-Br-furyl); C, 2-thienyl;
G, 3-pyridyl, M, 2-Cl-(pyrid-3-yl)
Ar: a, Ph; b, 2,4,6-Cl3C6H2
3.3. Antiarthropodal Activity
Several N-aryl benzenecarbohydrazonoyl chlorides I were reported active against anthropod pests and worms [29]. For example, N-(4-methylthiophenyl) benzenecarbohydrazonoyl chloride IB, N-phenyl 4-methylthiobenzenecar-bohydrazonoyl chloride ICa and its N-(2,4-dibromophenyl) analog ID [30] and N-phenyl 3-trifluromethylbenzocar-bohydrazonoyl bromide ICb [31] were found useful for control of anthropodal pests such as insects, spiders, ticks and mites.
IB, Ph-C(Cl)=NNH C6H4-SMe-4
ICa, 4-MeSC6H4-C(Cl)=NNHPh
ICb, 3-F3CC6H4-C(Cl)=NNHPh
ID, 4-MeSC6H4-C(Cl)=NNH C6H3Br2-2,4
3.4. Antiviral Activity
A series of N-aryl 2-aryl-2-oxo-ethanehydrazonoyl bromides VII were tested as antiviral agents. The results showed that all compounds investigated succeeded to reduce the number of local lesions induced by tomato mosaic virus on detached Datura metel leaves [32]. The order of activity was found to be Y/X: 4-Br / H = H / 4-Cl > H / 4-Me = 4-Me /H > H / 4-NO2. Analysis of the results obtained revealed that the activity of the studied compounds is more than that found for N-(4-nitrophenyl) benzenecarbohydrazonoyl bromide I. This result was considered to indicate that the presence of the 2-oxo group enhances the antiviral activity [32].
VII YC6H4-CO-C(Br)=NNHC6H4X
Y/X: a, 4-Br / H; b, H / 4-Cl; c, H / 4-Me; d, 4-Me /H; e, H / 4-NO2
3.5. Antimicrobial Activity (Fungicidal and Bactericidal)
Twenty seven hydrazonoyl halides of types I, III and VI were examined for their toxicity, fungicidal, fungistatic and bactericidal activities. The fungicidal activity was found to depend on the acid residue R [33]. The toxicity of the studied halides I, III and VI in warm blooded animals decrease in the order of substituents H > 4-NO2 > 4-Cl > 4-Br > 2- or 3-NO2 [33, 34].
I, YC6H4-C(X)=NNHC6H3R'R"-2,4
Y = H, 4-Br, 4-F, 4-Cl, 3-O2N, 4-O2N; X = Cl, Br
III, R-C(X)=NNHC6H3R'R"-2,4
R: Me, Et, Pr; X: Cl, Br
VI, MeCO-C(Cl)=NNHC6H3R'R"-2,4
R' = H, Br, O2N; R" = H, Br, Cl, O2N, H2NSO2
Dubenko et al. [35] reported that some N-aryl derivatives of, 2-oxo-2-phenylethanehydrazonoyl chlorides VII, 2-amino-2-oxoethanehydrazonoyl chlorides IX and cyanomethanehydrazonoyl chlorides XIII showed activity against wheat stem rust, phytophora infection of tomatoes and cucumber powdery mildew. Both systemic and contact activities were reported. Activities of these compounds were correlated with their structure.
VII PhCO-C(Cl)=NNHC6H4X
IX ArNHCO-C(Cl)=NNHC6H4X
XIII NC-C(Cl)=NNHC6H4X
X: a, H; b, Cl; c, Br; d, I; e, O2N; f, AcNHSO2; g, EtOCO
Also some N-aryl 2-oxopropanehydrazonoylchlorides VI were reported to be useful fungicides [36].
VI, CH3COC(Cl)=NNHAr; Ar = RR'C6H3,
R = H, 2-Cl, 2-Me, 3-F3C
R' = H, 2-, 3-, 4-Cl, 5-F3C, 6-Me, 4-O2N
3.6. Phytotoxicity Activity
Kukota et al. [37] tested fifteen N-(4-substituted phenyl) 3-bromo-2-oxopropanehydrazonoyl bromides VI and their 3-pyridinio bromide analogs IV as plant growth regulators at 0.01, 0.001 and 0.0001 %. The N-(4-methylphenyl)-, N-(4-chlorophenyl)- and N-(4-nitrophenyl)- derivatives depressed the growth of roots, stalks and leaves of lettuce and Oats.
VI, BrCH2COC(Cl)=NNHC6H4X
IV, [C5H5N+-C(Cl)=NNH C6H4X] Br-
X = 4-Me, 4-Cl, 4-O2N
3.7. Herbicidal Activity
Kaugars and his coworkers [27, 28] prepared two series of N-aryl hetarylcarbohydrazonoyl chlorides IV and indicated that they are primarily useful as herbicides.
IV, Het-C(Cl)=NNHAr
Het: A, 2-furyl, B, 2-(5-Br-furyl), C, 2-thienyl, D, 2-picolinyl,
E, nicotinyl, F, 4-pyridinyl
Ar = XC6H5-n; X: a, H, b, 2,4,6-Cl3
N-(2,4-Dichlorophenyl) propanehydrazonoyl chloride IIIa and its N-(2,4,6-trichlorophenyl) analog IIIb were reported to have, at 1Ib/acre, postemergence contact herbicidal activity against bread leaf weeds, and to a lesser degree against grasses [38]. Some other N-aryl (C2-4)alkane-hydrazonoyl chlorides IIIA were reported to be useful herbicides [39].
CH3CH2-C(Cl)=NNHAr
III, Ar: a, 2,4-Cl2C6H3; b, 2,4,6-Cl3C6H2
IIIA, R-C(Cl)=NNHC6H5-nXn
R = C2-4; X = H, 4-Cl, 2,4-Cl2, 4-Me
Also a series of N-aryl cyanomethanehdrazonoyl chlorides XIII was prepared and tested on lettuce and Oat cultured on agar [40]. Of these hydrazonoyl chlorides only N-(4-nitrophenyl)- and N-(2,4-dichlorophenyl)- derivatives XIIIa and XIIIb were reported to be the most effective herbicides, decreasing germination and inhibiting growth to the highest extent. Both compounds acted as herbicides at 0.0001% [40]. N-(4-methylphenyl) cyanomethanehydrazonoyl chloride XIIIc at 0.0001% was reported to stimulate lettuce stem growth to the highest extent [40].
XIII, NC-C(Cl)=NNHAr
Ar: a, 4-O2NC6H2; b, 2,4-Cl2C6H3; c, 4-MeC6H4
Twelve N-aryl alkanehydrazonoyl chlorides IIIA were also prepared and found useful as herbicides (e.g., against crabgrass, wild oats, yellow foxtail) [41]. N-(4-Chloro-phenyl) 2,2-dimethylpropanehydrazonoyl chloride IIIB was reported to exhibit the highest activity against crabgrass, wild oats and yellow foxtail [41].
IIIA, R-CX)=NNHAr, R = Et, Pr, Me2CH, Me3C
IIIB, (CH3)3C-C(Cl)=NNHC6H4Cl-4
Some other derivatives of N-Phenyl benzenecarbohydrazonoyl chloride I were prepared as useful herbicides [42]
I, XC6H4-C(Cl)=NNHC6H4Cl-2
X: alkyl, alkoxy, alkenyl, halo, CN, O2N
3.8. Antisarcoptic Activity
Strinadkin et al. [43] reported that of the twenty derivatives of N-aryl substituted benzenecarbohydrazonoyl-, eth-anehydrazonoyl-, 3-pyridinecarbohydrazonoyl-, 4-pyridine-carbohydrazonoyl- and 2-oxopropane-hydrazonoyl halides I-VI, respectively N-(2-nitrophenyl) benzene-carbohydra-zonoyl chloride was the most effective as antisarcoptic. A single application (5-10 ml 0.05%) of such a chloride or 0.5% of N-(2,4-dibromophenyl) benzenecarbohydrazonoyl bromide I to rabbit's ears eradicated psoroptosis within 10 days.
I Ar-C(Cl)=NNHAr'
III CH3-C(Cl)=NNHAr
IV Het-C(Cl)=NNHAr
VI CH3CO-C(Cl)=NNHAr
Het: F, 4-Pyridyl G, 3-Pyridyl
Also, Buzykin et al. [44] studied the antisarcoptic activity of twenty one hydrazonoyl halides of types I, III and X against Psoroptos Cuniculi and reported that the activity of I increases with increasing electron donating properties of the substituent in the Ar’ group, 4-MeO > 4-Cl > 4-Br > 3-NO2 > 4-NO2 > 2-NO2. They found that one application of 0.05% of a 13.6% compositon emulsion of N-phenyl benzenecarbohydrazonoyl chloride Ia to rabbit ears 100% eradicated psoroptosis. LD50 of hydrazonoyl halides studied for mice was 0.4-13.5 ml/kg vc. a 0.5-10.9M LC50 for P. Cuniculi. Both N-(2-nitrophenyl) benzenecarbohydrazonoyl chloride Iband N-phenyl 4-bromobenzenecarbohydrazonoyl chloride Ic were strongly allergenic to rabbits.
I, Ar-C(Cl)=NNHAr'
Ar' = XC6H4; X = 4-MeO, 4-Cl, 4-Br, 3-NO2, 4-NO2, 2-NO2
III, HC(Cl)=NNHAr'
X, (RO)2PO-C(Cl)=NNHAr'
3.9. Acaricidal and Miticidal Activity
N-Phenyl 4-methylthiobenzenecarbohydrazonoyl chloride Ia, N-(4-methylthiophenyl) benzenecarbohydrazonoyl chloride Ib and N-(2,4-dibromophenyl) 4-methylthioben-zenecarbohydrazonoyl chloride Ic [24, 25, 45] and N-(4-chlorophenyl) 3,3-dimethyl-2-oxopropanehydrazonoyl chloride VI [41] were reported to be useful as acaricides and miticides.
I Ar-C(Cl)=NNHAr'
VI Me3CCOC(Cl)=NNHC6H4Cl-4
Ar / Ar': a, H/ 4-MeSC6H4; b, 4-MeSC6H4 / H;
c, 4-MeSC6H4/2,4-Br2C6H3
Kaugars et al. [46, 47] screened seventy N-aryl benzenecarbohydrazonoyl chlorides I for miticidal activity and repellency, using the two-spotted spider miet. They reported that such activities depend to a large extent on the nature, position and number of substituents in either or both aromatic rings, the highest activity was shown by N-(2-chlorophenyl) derivatives of 4-chloro- and 4-bromo-benzenecarbohyd-razonoyl chlorides Ia,b and N-(3-trifluoromethyl) benzenecarbohydrazonoyl chloride Ic. Replacement of the hydrazone NH by N(CH3) decreased the activity [48].
I Ar-C(Cl)=NNHAr'
Ar / Ar': a, 4-ClC6H4 / 2-ClC6H4H; b, 4-BrC6H4/ 2-ClC6H4; c, C6H5 / 3-F3CC6H4
Kaugers and Germrich [15, 49] indicated that N-aryl benzene-carbohydrazonoyl chlorides I are highly active as miticides. One of these compounds, namely N-(2,4,6-trichlorophenyl) benzenecarbohydrazonoyl chloride IB, has been extensively field tested and has been assigned the trade mark Banomite. The latter was reported to decrease significantly Texas citrus mite (Euteranychus banksi) eggs and adults [50]. Other derivatives of benzenecarbohydrazonoyl chloride having methylthio group in either the C-phenyl or the N-phenyl groups were reported to be useful as acaricides [25].
IB Ph-C(Cl)=NNHC6H2Cl3-2,4,6
Several other N-aryl alkanehydrazonoyl chlorides III were also reported to be useful as acaricides [39, 51].
III RC(Cl):NNHAr
R = Me, Et, n-Pr, i-Pr, t-Bu
Ar = 2,4-Cl2C6H3; 4-Cl,2-NO2C6H3, 2,4,6-Cl3C6H2
Also, N-phenyl methylthio-substituted benzenecarbohydrazonoyl chlorides IA,B were useful as acaricides [24, 52].
IA,B RC6H4C(Cl):NNHC6H4R’
R/R’: 4-MeS / H, 4-Cl,2-MeS / H, H / 4-MeS, 4-MeS / 2,4-Br2
N-(2,4,6-Trichlorophenyl) benzenecarbohydrazonoyl chloride IB (banamite) was found more toxic (LC50 12 ppm) to the two spotted spider mite (Tetranychus urticae) than were nine of its potenrial metabolites. It inhibited rat liver monoamine oxidase at medium inhibitory concentrations (I50) of 4.7 x 10-5, 1.2 10-4 > 1.0 x10-3 and 1.4 x 10-3 M, respectively. Compounds relatively nontoxic to mites were usually ineffective monoamine oxidase inhibitors [53].
IB, Ph-C(Cl)=NNHC6H2Cl3-2,4,6
In another report [54], it was indicated that the acaricidal activity of the dithioketal of N-(2,4-dichlorophenyl) 2-oxopropanehydrazonoyl chlorides IVA against the 2-spotted spider mite (Tetranychus urticae) on lima bean plants depended on R group, and was in the descending order R: Me, Et, Ph, H and C5H11, that is the most active compound thus being the chloride IVAa. The thioketal IVAa was reported to be much more active than the corresponding ketal IVBa. Of the series IV, the most active halides were those having 3-F3C, 4-Brsubstituents in the N-aryl group.
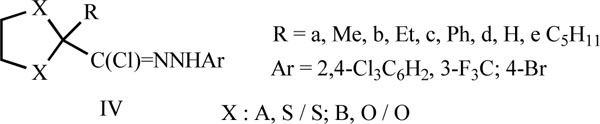
Emmel et al. [55, 56] prepared nineteen derivatives of ethyl N-arylhydrazonochloroacetates VIIIA and their methyl analogs VIIIB and used against red spiders on beans and apple trees. Also, N-(2,4-dibromophenyl) benzenehydrazonoyl bromide I was reported to be effective gave 100% control of houseflies in 48 h [57].
VIII ROCOC(Cl):NNHC6H4X
I PhC(Br)=NNHC6H3Br2-2,4
R: A, Et; B, Me
X: H, 3-F3CO, 3-FCH2-CF2-O, 3-Cl2CH-CF2-O, 2-Me-4-Cl2CH-CF2-O
N-Phenyl and N-(2,4,6-trichlorophenyl) derivatives of 2-furyl-, 2-thienyl, 2- or 3-pyridyl- and 2-chloro-4-pyridyl- carbohydrazonoyl chlorides IV were also proved to have acaricidal activity [58].
IV Het-C(Cl)=NNHAr
Het: A, 2-furyl; B, 5-Br-2-furyl; C, 2-thienyl; G, 3-pyridyl;
H, 2-pyridyl; I, 2-Cl-4-pyridyl;
Ar: Ph, 2,4,6-Cl3C6H2
Also, eighteen N-aryl 2-oxopropanehydrazonoyl chlorides VI were prepared and found useful acaricides [36].
VI, CH3COC(Cl)=NNHC6H3RR'
R = H, 2-Cl, 2-Me, 2-Et R' = H, Me, Et
N-(4-Fluorophenyl) 2-chlorobenzenecarbohydrazonoyl chloride IC was prepared and reported to control totally Plutella maculipennis larvae on cabbage leaves when 0.01% concentration of it was used [42].
IC, 4-F-RnC6H4-nC(Cl)=NNHC6H5
R = alkyl, alkoxy, alkenyl, halo, CN, O2N; n = 0, 1-4
Also, thirty four alkanehydrazonoyl chlorides III, IV, IB and V were found useful as acaricides and insecticides [27, 28, 59-64].
III, R-C(Cl)=NNHAr
R = CEmmel1-7 alkyl; Ar = 2-Cl-4-R”-5-R’C6H2: R’ = C1-4 alkoxy, CH2=CHCH2O, HC=CCH2O, or Cl, R” = Cl, Me or HC=CCH2O
IV, Het-C(Cl)=NNHAr;
Het: A, 2-furyl, B, 5-Bromo-2-furyl; C, 2-thienyl, D, picolinyl, E, nicotinyl; I, 2-Cl-4-pyridyl;
Ar: Ph, 2,4,6-Cl3C6H2
IB, PhC(Cl)=NNHC6H2Cl3-2,4,6
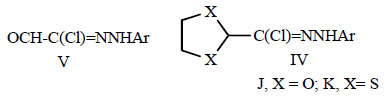
V, OCH-C(Cl)=NNHC6H3Cl2-3,4
3.10. Pesticides
N-Aryl 2-oxoethanehydrazonoyl chlorides V and their ketal and thioketal derivatives IV were reported to have strong pesticidal effects [65].
Kaugars et al. [46, 66]reported that various substituted N-phenyl benzenecarbohydrazonoyl chlorides I have pesticidal properties.
I, Ar-C(Cl)=NNHAr'
N-Phenyl 3-trifluoromethylbenzenecarbohydrazonoyl chloride IAa was found by Kaugars et al. to be useful pesticide for controlling arthropods [31]. In addition, it was reported that N-phenyl 4-methylthiobenzenecarbohydrazonoyl chloride IAb and N-(4-methylthiophenyl) benzenecarbohydrazonoyl chloride IBa are useful for control of arthropodal pests such as insects, spiders, ticks and mites [63].
IAa, 3-CF3C6H4-C(Cl)=NNHPh
IAb, 4-MeSC6H4-C(Cl)=NNHPh
IBa, Ph-C(Cl)=NNHC6H4SMe-4
3.11. Insecticidal Activity
Eighteen N-aryl 2-oxopropanehydrazonoyl chlorides VI were prepared and reported to be useful insecticides [36].
VI, MeCO-C(Cl)=NNHC6H3RR'
R: H, 2-Cl, 2-Me, 3-F3C
R': H, Me, Et
N-Phenyl and N-(2,4,6-trichlorophenyl) derivatives of 2-furan-, 2-thiophene-, 2-pyridine-, 3-pyridine- and 2-chloro-4-pyridine- carbohydrazonoyl chorides IV were found useful as insecticides because of their insect metamorphosis-inhibiting activity [27, 58, 59]. Tests on cabbage looper and three other insects were given.
IV Het-C(Cl)=NNHAr
Het: A, 2-furyl; C, 2-thienyl; G, 3-pyridyl; I, 2-Cl-4-pyridyl
Ar: Ph, 2,4,6-Cl3C6H2
N-Phenyl 2-substituted-propanehydrazonoyl chlorides of type IIIA and IIIB were also reported to be useful as insecticides because of their insect metamorphosis-inhibiting activity [41, 67].
IIIA, Me-CH(SR)-C(Cl)=NNHPh
R = Me, Et, i-Pr, t-Bu, 4-ClC6H4
IIIB, R'C(Cl)=NNHAr
R': Et, Pr, Bu, i-Pr, t-Bu
Ar = XC6H4; X: H, 2-Me, 4-O2N, 4-Cl, 2,4-Cl2, 2,4,6-Cl3
In addition, substituted N-phenyl benzenecarbohydrazonoyl halides I and N-aryl (C2-4)-alkanehydrazonoyl chlorides III were reported to be useful as insecticides and miticides [25, 39, 46, 49, 66]. For example N-(2,4-dibromophenyl) benzenecarbohydrazonoyl bromide 1 gave 100% control of houseflies in 48 h.
I, XC6H4-C(Cl)=NNHC6H4Y
X: H, 3-, 4-Me, 4-F, 2-, 3-, 4-Cl, 4-Br, 4-I, 2-Me, 4-i-Pr, 2,4-Cl2, 3,4-Cl2, 2,6-Cl2, 2,4-Cl(NO2), 3,4-Me(NO2), 3,4-Me2, 2,5-Me2, 3,5-Me2, F5
Y: 2,4-Br2, 2,5-Cl2. 2-Me, 4-Cl, 4-O2N,
III, R-C(Cl)=NNHAr
R = CnHn-1, n = 2,3,4
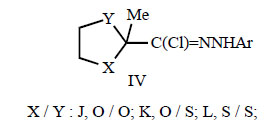
Furthermore, ketal, thioketal and dithioketal derivatives of N-aryl 2-oxopropanehydrazonoyl chlorides IVJ-L were reported to be useful insecticides for European red mites and double-spotted spider mites [65, 68].
N-Phenyl and N-(4-methylthiophenyl) derivatives of benzenecarbohydrazonoyl chloride and its 2- and 4-methylthio analogs I were also reported to be useful as insecticides and acaricides [15, 24, 42, 52].
I, Ar-C(Cl)=NNHAr'
Ar = Ph, 2-MeSC6H4, 4-MeSC6H4
Ar' = Ph, 4-MeSC6H4
Kaugars prepared a series of N-aryl C-heteroaryl-methanehydrazonoyl chlorides IV and found that such halides are useful as insecticides [28, 60].
IV, Het-C(Cl)=NNHC6H5-nCln
Het: A, 2-furyl, C, 2-thienyl, D, 2-pyridyl, G, 3-pyridyl, I, 4-pyridyl n = 1, 2, 3
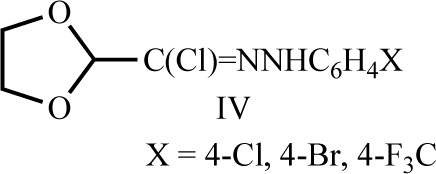
Also, N-(2,4-dibromophenyl) benzenecarbohydrazonoyl bromide Ie was reported to be effective as insecticide as it gave 100% control of house flies in 48 hr [57].
I, Ph-C(Br)=NNHC6H3Br2-2,4
In another report, it was indicated that the ketal derivatives of N-aryl 2-oxoethanehydrazonoyl chloride IV are effective insecticide [69].
Also, N-(4-chlorophenyl) 2,2-dimethylpropanehydrazonoyl chloride III was found useful as insecticide against housefly, cotton weevil and Mexaican beanbeetle [41].
III (CH3)3C-C(Cl)=NNHC6H4Cl-4
Also, several N-(2,4,6-trichlorophenyl) 2-alkylthioalkane-hydrazonoyl chlorides III were found to be useful as insecticides having morphogenic hormonal mimetic activity [70-72]. The bis-hydrazonoyl chlorides X1 were reported to have insecticidal activity against cabbage looper and alfafa weevil larvae [39].
The bis-hydrazonoyl chloride XII was reported to be useful insecticide for fertilizers [73].
3.12. Weed Controlling Agents
Kaugars [41] indicated that tweleve N-aryl (C3-5)alkane hydrazonoyl chlorides III are useful as miticides, insecticides against housefly, cotton ball weevi, mexican bean beetle and as herbicides against crabgrass, wild oats and yellow foxtail.
III, R-C(Cl)=NNHAr; R = CnHn-1, n = 3,4, 5
In addition, Moon [74] prepared a series of 1'-formyl-4'-halobenzeneazamethanes VI and indicated that they are useful as weed controlling agents.
VI, RCOCR'(X)-N=N-Ar;
R = Lower alkyl, cycloalkyl, cycloalkoxy, haloalkyl, haloalkoxy
R' = Lower alkyl, cycloalkyl, Ph
X = Br, Cl
The hydrazonoyl chlorides VII, IX and XIII showed also activity against wheat stem rust, phytophora infection of tomatoes and cucumber powdery mildew [34].
VII, PhCO-C(Cl)=NNHAr IX, H2NCO-C(Cl)=NNHAr
XIII, NC -C(Cl)=NNHAr
In addition, the hydrazonoyl bromides VI were tested as plant growth regulators. They depressed the growth of roots, stalks and leaves of lettuce and oats [37].
VI BrCH2COC(Br)=NNHC6H4X
X = 4-Me, 4-Cl, 4-NO2
3.13. Lipoxygenase and Cyclooxygenase Inhibitors
N-(2,4,6-Trichlorophenyl) benzenecarbohydrazonoyl chloride (banamite) IB was reported to be more toxic (LC50 12 ppm) to the twospotted spider mite (Tetranychus urticae) and it inhibits rat liver manoamine oxidase at median inhibitory concentrations [53]. Compounds relatively nontoxic to the mites were usually ineffective monoamine oxidase inhibtors
IB PhC(Cl)=NNHC6H2Cl3-2,4,6
4. METABOLIC FATE
The metabolism of the acaricide Banamite (N-(2,4,6-trichlorophenyl) benzenecarbohydrazonoyl chloride) was reported. It was indicated that it is metabolized slowly by the two-spotted spider mite (Tetranychus urticae). The major metabolites identified were benzaldehyde N-(2,4,6-trichloro-phenyl)hydrazone and benzoic N-(2,4,6-trichloro)hydrazide. Minor metabolites identified were 2,4,6-trichloroaniline, benzaldoxime, 2,4,6-trichlorophenylhydrazine and benzoic acid [61].
Also, the metabolism of one of the anthelmintic hydrazonoyl halides was studied in sheep and rats by Jaglan and coworkers [75-79]. For this purpose, the authors used 14C-(Phenylhydrazine) and 14C-(carboxy) labeled N-phenyl p-toluenecarbohydrazonoyl chloride (TCPH-I) and (TCPH-II), respectively. Ten days after a single oral therapeutic dose of 50 mg/kg, 93% of the radioactivity was recovered, 19% in urine and 74% in feces. The 14C residues were higher and persisted longer in blood and blood rich organs such as liver, lung, kidney and spleen compared to other tissues. The 14C residues were largely present in the hemoglobin. Such residues could neither be extracted into organic solvents nor separated from hemoglobin by dialysis, gel filtration or electrophoresis. Administration of TCPH-II resulted in a lower concentration of 14C in the blood. Most of the 14C residue in the blood was found in the plasma rather than in the erythrocytes which demonstrated that only the phenylhydrazine part of the molecule was bound to erythrocytes. Chromic acid oxidation of heme or globin from TCPH-I experiment produced 14C-benzoic acid. This finding was considered to indicate that the phenyl part of TCPH was bound to hemoglobin and that the carboxyl carbon of benzoic acid comes from heme or globin [75].
In another report [76], it was indicated that both labels TCPH-I and TCPH-II cleared the gastrointestinal tract of treated sheep within ten days with the fecal radioactivity levels being 3-4 times greater than those for urine. Fractionation of blood from TCPH-I treated sheep showed that the majority of the radioactivity (66%) was associated with protein with erythrocytes having ten times the radioactivity of plasma. Although plasma levels were approximately equal for both TCPH-I and TCPH-II, 14C levels in erythrocytes from the form TCPH-I treatment were 15 times greater than with TCPH-II treatment suggesting cleavage of the molecule with only phenylhydrazine moiety being retained. Erythrocytic 14C was bound to hemoglobin [76].
An analytical procedure, to measure the level of phenyl groups incorporated in heme, based on their oxidation to benzoic acid, was developed to monitor the residues in treated animals [77]. Relay metabolism in rats was studied by feeding sheep blood containing 14C residues from 14C-TCPH treatment. No retention of 14C residues in rat tissues was observed, which contrasted with the TCPH metabolism. A 90-day relay toxicity study in rats, which were fed dried blood from treated sheep containing up to 2000 times the potential exposure to residues in human diet, indicated no observable toxic responses. It was concluded tat these data support a tolerance of 6 ppm TCPH equivalents in blood [77].
Furthermore, it was indicated that benzene was characterized as a volatile metabolite of p-toluic acid phenylhydrazide in rats. The relationship of benzene as the volatile metabolite of p-toluic acid phenylhydrazide and the phenyl groups bound to haemoglobin from treatment of sheep with 14C-(carboxy labeled) hydrazonoyl chloride (TCPH-I) was discussed [78].
In an attempt to identify the metabolites of TCPH in the urine and feces of treated sheep, Jaglan and coworkers [79] indicated that thin layer chromatography of ethyl acetate extracts of feces showed that about 12 % of the dose was present unchanged TCPH and < 2% as p-toluic acid phenylhydrazide (TAPH) and aniline. A major feal metabolite (27% of the dose) was characterized as 1-phenyl-1-acyl-2-p-tuolylhydrazine (where the acyl group was a mixture of stearyl, palmityl, myristyl and lauryl groups). Both TCPH and TAPH were not found in urine. Small amounts (< 1% of the dose) of p-toluic acid, α-ketoglutaric acid phenylhydrazone and pyruvic acid phenylhydrazone were also observed, based on cochromatography with synthetic compounds. The major urinary radioactivity (about 10 % of the dose) was characterized as p-methylhippuric acid, indicating molecular cleavage of TCPH [79].
5. CONCLUSION
From the previous literature survey, it is clear that hydrazonoyl halides are very useful chemical-biology tools. In addition, we now have in hand an impressive number of biologically active candidates that can be used for treatment of various diseases. Less attention, however, was directed toward the synthesis and biology of C-alditolyl hydrazonoyl halides which are expected to have more penetrating power in the living cells. Further studies along this line may lead to products with better biological properties. For those who will be interested in exploring the chemistry and biology of such class of hydrazonoyl halides, the various review articles by one of the authors and mentioned in the introduction will be of help. In the light of the present review, there is every reason to believe that additional new and important biological applications of hydrazonoyl halides are just waiting to be discovered. It is hoped that this review will further stimulate interest in the biology of this class of organic compounds.




